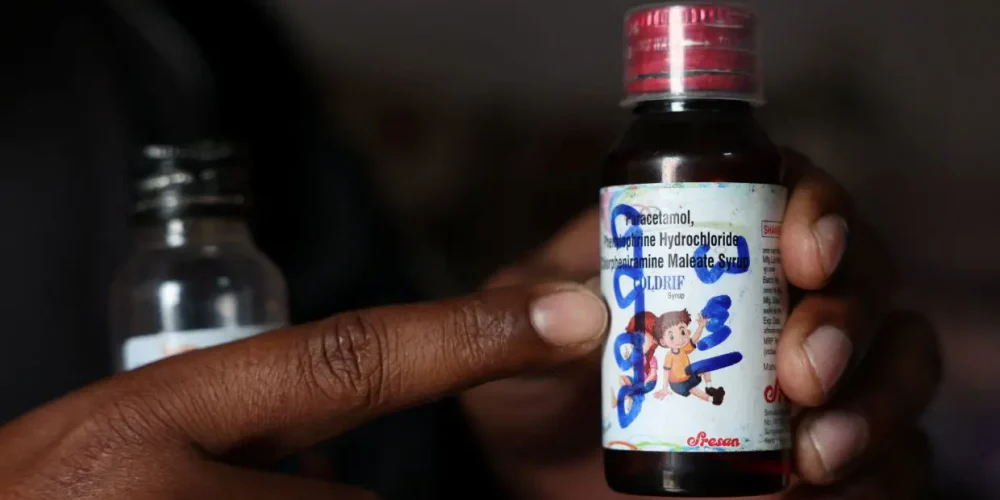
A series of tragic deaths linked to contaminated cough syrups from India has triggered growing global concern, spotlighting deep weaknesses in the country’s pharmaceutical oversight. Investigations reveal a troubling pattern of safety lapses, inadequate testing, and inconsistent regulatory enforcement that allowed toxic products to reach vulnerable children across several countries.
The crisis began when health authorities in multiple nations reported sudden and severe kidney injuries in children who had consumed Indian-made cough syrups. Laboratory testing later confirmed the presence of industrial-grade chemicals, including diethylene glycol and ethylene glycol—substances that can cause organ failure when ingested. These findings pointed to critical failures in manufacturing practices and quality control procedures at several Indian pharmaceutical plants.
India is one of the world’s largest suppliers of generic medicines and exports to more than 150 countries. But the recent incidents have exposed gaps in monitoring and compliance, particularly among smaller manufacturers. Experts say these companies often operate with limited supervision, outdated equipment, and insufficient safety protocols. While India’s drug laws require strict adherence to quality standards, enforcement varies widely between regions, leaving room for dangerous shortcuts.
Regulators in affected countries have taken swift action by banning specific cough syrups, issuing alerts, and recalling products. International bodies, including the World Health Organization, have also urged stronger oversight and emphasized the need for standardized global safety practices. The repeated incidents have prompted calls for an overhaul of India’s regulatory system to prevent similar tragedies in the future.
Investigations suggest that some manufacturers used contaminated solvents or failed to verify the purity of ingredients before formulating syrups. In several cases, the required testing either did not occur or lacked proper documentation. Industry analysts say these failures reflect systemic issues in the supply chain, where limited transparency makes it difficult to trace the source of contamination.
Parents in affected communities describe devastating loss, while health professionals warn that the full impact may extend beyond the reported cases. Many families in low-income regions rely on imported or donated medicines without the ability to verify safety, making them more vulnerable when regulatory checks break down.
India’s government has acknowledged the crisis and launched inspections across multiple facilities. Some plants have been shut down, and legal action is underway against companies accused of violating safety norms. Officials say they plan to strengthen testing requirements, improve licensing procedures, and expand regulatory manpower to ensure compliance across the industry.
The tragedy has renewed debate about the global dependence on low-cost pharmaceuticals. While India supplies affordable medicines to millions, stakeholders argue that cost should not come at the expense of safety. Experts believe the solution lies in stronger oversight, transparent supply chains, and better international coordination to ensure consistent quality standards.
Global health advocates warn that restoring trust will require significant reforms. For now, the crisis stands as a stark reminder of how weak oversight in one part of the world can lead to catastrophic consequences elsewhere. As investigations continue, families and policymakers are left demanding accountability and urgent reforms to prevent future tragedies.
Read More : WHO Urges India to Strengthen Oversight on Toxic Cough Syrups After Child Deaths