AI Helps Reveal How New Antibiotic Targets Gut Bacteria
Researchers at MIT’s CSAIL and McMaster University have used generative AI to accelerate a key step in antibiotic development: figuring out exactly how a drug molecule interacts with its bacterial target. Their work could help usher in more precise, less damaging treatments for gut infections.
🎯 Enter
Enterololin
: A Narrow-Spectrum Antibiotic
The team discovered a compound named enterololin, which specifically suppresses E. coli strains linked to inflammatory bowel disease (IBD) flare-ups — while largely sparing the rest of the gut microbiome. In mouse models, animals treated with enterololin recovered more quickly and maintained healthier microbial balance than those given a traditional antibiotic like vancomycin.
What makes enterololin especially intriguing is its precision: it doesn’t act like a broad-spectrum antibiotic that can indiscriminately wipe out beneficial bacteria.
🧠 Using AI to Crack the Mechanism of Action
One of the biggest bottlenecks in antibiotic development is identifying how a molecule acts inside a bacterium — what protein it binds, and how that affects the cell’s function. This process traditionally takes 1 to 2 years or more.
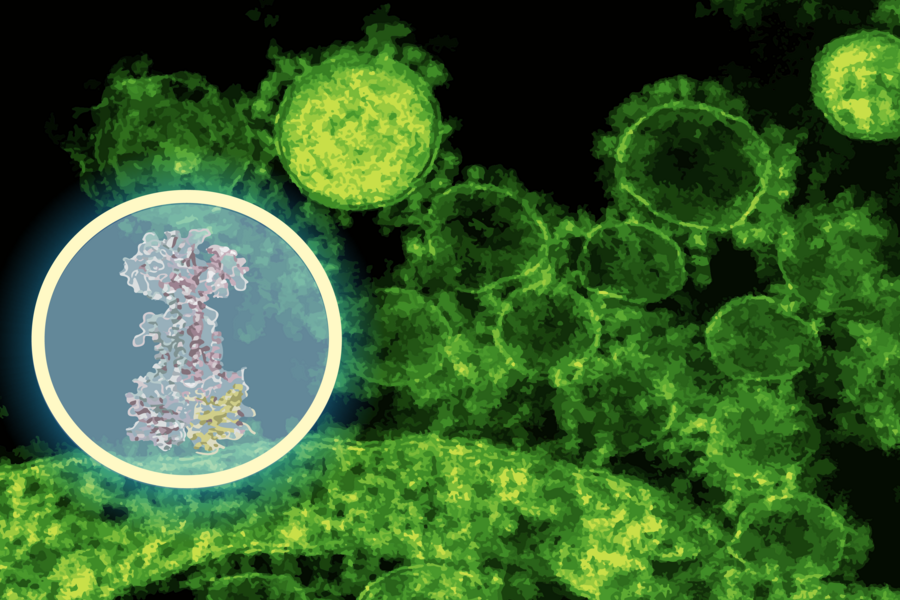
To speed this up, the researchers used an AI model called DiffDock (developed at MIT) to predict how enterololin might bind to bacterial proteins. Within minutes, DiffDock suggested that enterololin binds to a protein complex called LolCDE, which is essential for lipoprotein transport in certain bacteria.
The team then validated this prediction via lab experiments: creating resistant bacterial mutants, running RNA sequencing, and using CRISPR knockdowns. All data pointed toward disruptions in the lipoprotein transport pathway — the same pathway predicted by the AI.
Because the AI’s prediction matched the experimental results, the researchers believe they’ve cracked the molecule’s mechanism of action much faster than would normally be possible.
🌐 Implications & Future Steps
- Faster drug development: This AI-guided approach reduced a process that might take years down to about six months.
- Precision antibiotics: Tools like enterololin may help us move away from blunt, broad-spectrum antibiotics and toward targeted therapies that avoid collateral damage to harmless or helpful microbes.
- Beyond gut bacteria: The research team is already exploring derivatives of enterololin to target other hard-to-treat pathogens like Klebsiella pneumoniae.
- Commercial development: A startup called Stoked Bio has licensed enterololin and is optimizing it for human use, with hopes of advancing to clinical trials in the coming years.
This work signals a shift in how AI is used in life sciences: not just for screening molecules, but for explaining how molecules act — thereby helping bridge the gap between lab discovery and safe, effective therapies.


